LLM Fine-tuning & Classification Development
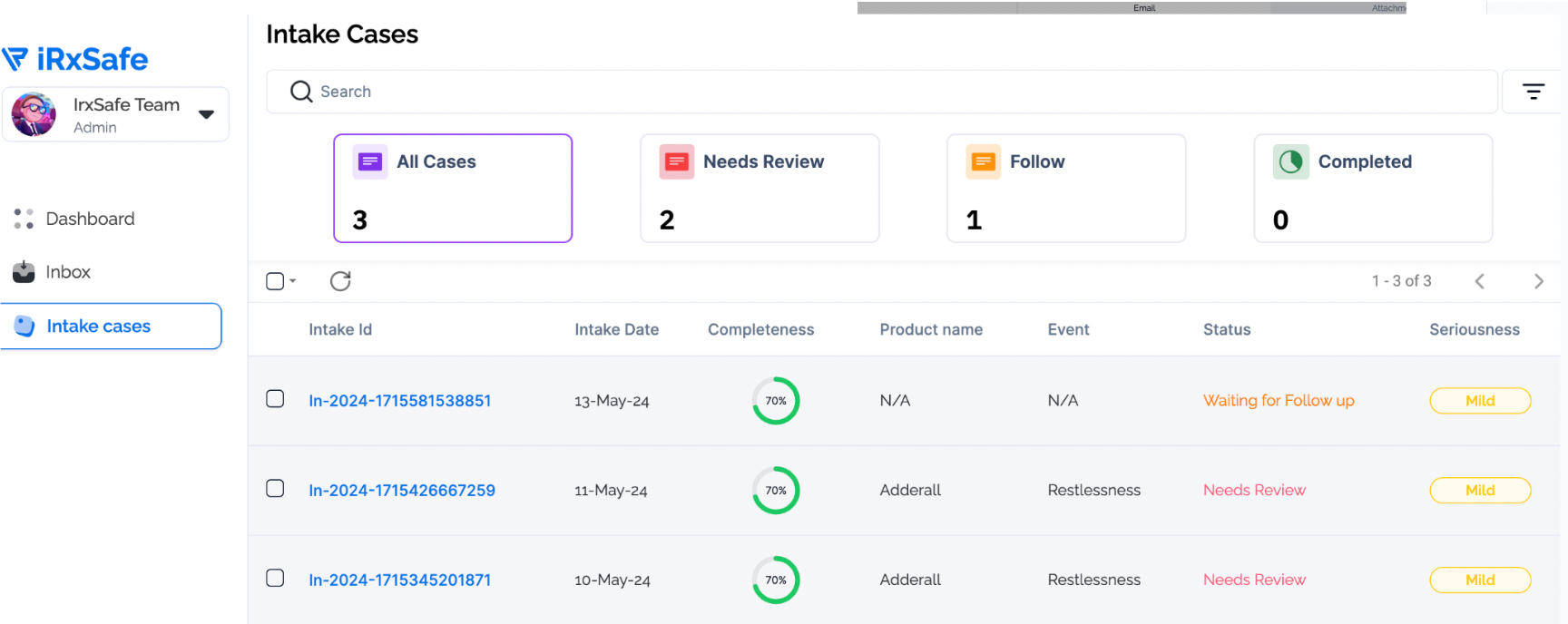
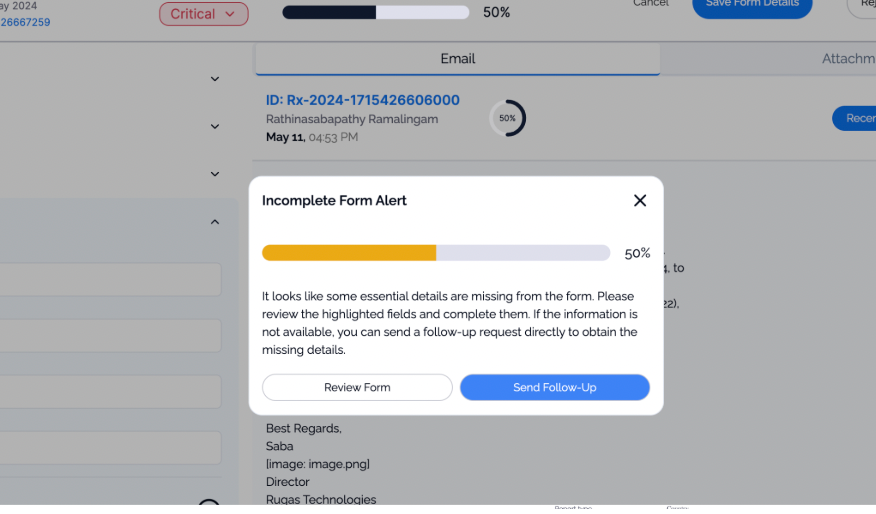
Fine-tuned Claude Sonnet language model on pharmaceutical safety data, adverse event reports, and medical terminology for accurate email classification and information extraction.
Developed multi-class classification system distinguishing safety reports, product quality issues, and general correspondence with high precision for appropriate routing and processing.